 |
What is causing the ocean's acidification?
Ocean waters are constantly reacting with environmental gasses. In particular, the oceans absorb up to half of the carbon dioxide in the atmosphere. They play a key role in the carbon cycle too, by acting as a carbon sink (store).
In this case, the upper level or layer of the ocean or seawater (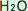 ) absorbs ) absorbs 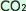 , forming Carbonic acid. The chemical symbol for carbonic acid is , forming Carbonic acid. The chemical symbol for carbonic acid is  . .
Here is the chemical equation:
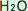 + + 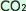 = = 
The result of this reaction increases Hydrogen ions in the water, and reduces carbonate ions. The reaction also leads to a reduction of the pH by about 0.1 units. Remember that the lower the pH, the more acidic the water is.
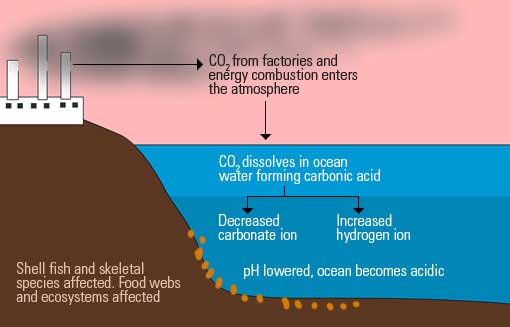
Note that the margin may look small, but the effect on living organisms depending on carbonate ions is very significant.
The IPCC forecasts that ocean pH will fall by "between 0.14 and 0.35 units over the 21st Century, adding to the present decrease of 0.1 units since pre-industrial times" *1
Water pH levels are not consistent across the globe. Some places such as the eastern Pacific have lower pH, whiles the arctic ocean area has a higher pH.
The above conditions are normal, but unfortunately, the earth has seen an increase in Anthropogenic 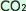 concentrations since the industrial revolution. (Anthropogenic concentrations since the industrial revolution. (Anthropogenic 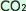 is the portion of atmospheric is the portion of atmospheric 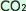 resulting from human activities such as burning fossil fuels) As a result, more resulting from human activities such as burning fossil fuels) As a result, more 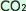 is absorbed by ocean water, as the equilibrium point has changed a bit. is absorbed by ocean water, as the equilibrium point has changed a bit.
 
|
 |

![]()